Vein Finder Assisted IVF check-up
In vitro fertilization (IVF) is a complex series of procedures used to help with fertility or prevent genetic problems and assist with the conception of a child.
Before undergoing an IVF operation, women are expected to do regular check-ups. These preparatory tests consist of Ovulation testing, blood testing, measuring hormone levels etc.
In order that these tests’ results, especially blood tests, be accurate and informative enough, professional vein finders ought to be used.
Several vein finding devices are being used by gynaecologists and nurses, yet, few of them proved to be efficient enough.
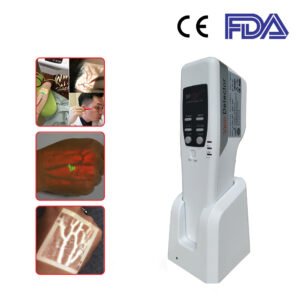
The FDA Portable Vein Detector SIFVEIN-5.2, however, has gained gynaecologists’ appreciation for many years.
The Vein Finder SIFVEIN-5.2 is a medical observation tool. It detects subcutaneous superficial veins by infrared light of research of development patent technology.
The SIFVEIN-5.2 Displays the situ image on the surface of the skin.
The ultimate aim is to help medical staff check women’s vascular orientation and distribution.
Further, the enhanced model has various colors, which can improve the clarity and efficacy of vein identification.
Relatedly, the device enjoys depth identification. That is, it provides a more specific vein location that can be used in the cardiovascular department for bypass surgery when accurate positioning of stripping veins is necessary. This should further improve the accuracy of clinical diagnosis.
With all these advanced options and even more, the Vein finder SIFVEIN-5.2 should be the most appropriate device that would facilitate IVF routinary check-ups. That, in turn, should pave the way for safe and successful IVF operations.
Reference: In vitro fertilization (IVF)
Disclaimer: Although the information we provide is used by different doctors and medical staff to perform their procedures and clinical applications, the information contained in this article is for consideration only. SIFSOF is not responsible neither for the misuse of the device nor for the wrong or random generalizability of the device in all clinical applications or procedures mentioned in our articles. Users must have the proper training and skills to perform the procedure with each ultrasound scanner device.
The products mentioned in this article are only for sale to medical staff (doctors, nurses, certified practitioners, etc.) or to private users assisted by or under the supervision of a medical professional.