Laser-Assisted Uvulopalatoplasty
Uvulopalatoplasty is a surgical procedure performed with the aim of reducing or eliminating snoring.
Laser-assisted uvulopalatoplasty (LAUP) has been used as a treatment option for snoring and obstructive sleep apnea for almost three decades.
It is an out-patient procedure, in which a laser is used to remove parts or all of the uvula at the rear of the mouth.
Uvulopalatoplasty non-surgical laser-guided operations often require the penetration of 980 nm laser wavelength so that the operation ends up being fruitful.
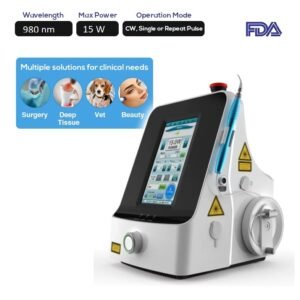
The FDA Portable 980nm Surgery Laser System SIFLASER-1.2B is specifically designed to meet that exact requirement as it functions through a 980 nm wavelength with 15W as maximum power.
These features are the exact needs in Uvulopalatoplasty non-surgical treatment/surgery.
To explain in more detail, the tissue components haemoglobin and melanin interact more with the blue laser light even at reduced power.
Accordingly, this allows SIFLASER-1.2B to perform better and softer bloodless, pain-free uvula cuttings.
What also makes the SIFLASER-1.2B highly recommended by surgeons is that it displays an accurate alignment and exact sighting during the surgery.
Further, in order to guarantee better results, the device is supported with a fiber guide laser.
First, it is compatible with various endoscopic uses. Second, this special fibres are sterilisable which, in turn, prevents any possible cross-infections while guaranteeing a clean and bloodless operative area that actually reduces all possible thermal damages.
Moreover, the complete absence of water absorption in the device helps drastically reduce the overheating of the surrounding tissues.
This should eliminate any possible after-effects which, in turn, will reinforce the laser treatment’s efficacy.
Due to all these features and more, the SIFLASER-1.2B guarantees increased uvula cutting effectiveness, much higher than the one obtained with infrared lasers.
The number of laser treatments in correlation to Uvulopalatoplasty has been constantly rising during the last years.
Nowadays, it is possible to carry out a vast number of Laser-guided bloodless Uvulopalatoplasty surgeries thanks to the FDA Portable 980nm Surgery Laser System SIFLASER-1.2B
Reference: Laser-assisted uvulopalatoplasty (LAUP) complications and side effects: a systematic review
Disclaimer: Although the information we provide is used by different doctors and medical staff to perform their procedures and clinical applications, the information contained in this article is for consideration only. SIFSOF is not responsible neither for the misuse of the device nor for the wrong or random generalizability of the device in all clinical applications or procedures mentioned in our articles. Users must have the proper training and skills to perform the procedure with each Laser System.
The products mentioned in this article are only for sale to medical staff (doctors, nurses, certified practitioners, etc.) or to private users assisted by or under the supervision of a medical professional.