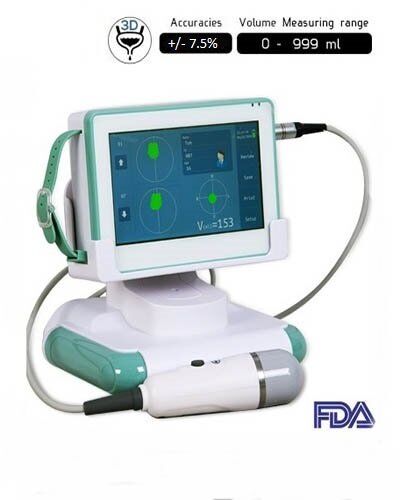
Urology Bladder Ultrasound scanner SIFULTRAS-5.54 FDA
$4,855
Volume Measurement Range : 0-999 ml.
Probe: 3D Mechanical Sector Scan.
Measurement Error : +/- 7.5%.
Intelligent Positioning and Pre-scanning Functions Available.
Bluetooth Wireless Transmission.
Printing mode: Yes
Certification: FDA.
For Quantity Discounts Please Call : +1-323 988 5889.
5 × Trees planted for one purchased item
Urology Bladder Ultrasound scanner SIFULTRAS-5.54 FDA
3D Bladder Ultrasound scanner SIFULTRAS-5.54 provides a virtual 3D image of the bladder and the volume of urine retained within the bladder. Retained urine is a reservoir for bacteria and pathogens, which can cause urinary tract infections, leading to damage of the renal structures, pain, and urosepsis.
3D Bladder ultrasound scanner SIFULTRAS-5.54 is commonly utilized in acute care, long-term care, and rehabilitation environments, as well as in physicians’ offices. Without the use of a bladder Ultrasound scanner, urinary retention is assessed by performing an invasive “in and out” urinary catheterization, which can be uncomfortable and pose a direct risk of introducing more pathogens into the bladder, increasing a patient’s risk of infection
SIFULTRAS-5.54 has various important features such as it can store 1000 cases and results with store and print function, plus it can synchronize patient information to the host computer.
Product Parameter:
- Volume Measurement Range: 0-999 ml.
- Probe: 3D Mechanical Sector Scan.
- Measurement Error: +/- 7.5%.
- Intelligent Positioning and Pre-scanning Functions Available.
- Scanning depth: ≥160 mm
- Store 1000 cases with store and print function
- Expert mode and simple mode for Choosing.
Urology Bladder Ultrasound scanner SIFULTRAS-5.54 FDA Specifications
| Equipment dimensions | 190*135*52 mm |
| Monitor size | 7-inch TFT-LCD |
| LCD resolution | 800*480 |
| Volume measurement range | 0 – 999 ml |
| Volume measurement accuracy | ±10 % (registered in FDA & CE) |
| ±7.5 % (registered in CFDA) | |
| +6 % (actual clinic test) | |
| Volume display resolution | 1 ml |
| Sceen type | Capacitive touch screen |
| Operation methods | Touch keyboard |
| Shortcut button | YES |
| Weight (within probe) | About 1300g |
| Information storage | 1,000 cases of patient record |
| Patient name input | 25 digits |
| Probe feature | 3D mechanical sector scan, no need to calibrate |
| Probe frequency | 2.5 MHz, B-mode |
| Scanning time | 5 seconds |
| Scanning angle | 120 degrees |
| Scanning depth | 190 mm Max |
| Gender selection | Male, Female, Child |
| Prescan function | YES |
| Calculation Trace | Automatic |
| Ultrasound diagnosis technology | Tissue harmonic imaging |
| Number of ultrasound images | 12 pictures |
| Image color selection | Grey/Yellow/Green/Blue/Aqua/Fuchsia |
| Interface theme selection | Blue/Green |
| Multi-language selection | English/Français/Dansk/Suomi/Português/Svenska/Español/Nederlands/Norsk/Deutsch |
| Operation mode selection | Expert mode/Easy mode |
| Information print | Built-in thermal printer |
| External memory | USB disk |
| PC connectivity | USB port/Bluetooth wireless |
| Power consumption | 50W |
| Power supply | AC 100 – 240 V, 50/60 Hz, 1.2 A |
| DC 13.5 V, 5 A | |
| Battery capacity | DC 11.1 V, 2600 mA |
| Battery charging time | less than 2 hours |
| Fully charged battery life | Continuous scan: more than 2 hours |
| Standby time: more than 4 hours |
Clinical Significance:
- To instruct the doctor or the patient to Manage the timing of the appropriate catheterization in order to reduce the patient’s diuretic pain.
- Reduce the risk of urinary infection caused by catheterization.
- Guide the evaluation of the recovery of bladder function before and after surgery of Urology, Gynecology, and Pelvis.
- Monitor the postvoid residual volume and prevent urinary retention.
- To monitor the bladder’s capacity in order to guide the pelvic accurate radiotherapy.
Applications:
Obstetrics and Gynecology Department,Radiotherapy Department,Urology Department,Rehabilitation Department, Neurology Department, ICU, Emergency Department, Endocrinology Department, Nursing and Rehabilitation Institution, Household Rehabilitation, Elderly Care Center, etc.
Wireless Bluetooth 3D Bladder Ultrasound scanner SIFULTRAS-5.54 FDA has an optional trolley
Certification:
FDA
CE.
ISO.

1 × Wireless Bluetooth 3D Bladder Ultrasound scanner SIFULTRAS-5.54 FDA
1 × Trolley (optional)
12-Month Warranty
![]()
![]()
![]()
![]()
![]()
5 × We plant for you ten Trees





× 5 Trees planted for one purchased item
One Tree Planted is on a mission to reforest our planet and provide education, awareness and engagement on the importance of trees in our ecosystem. It also has a social impact encouraging and giving incentive to low income people to plant Trees in their Area.
Reducing carbon footprint: A mature tree absorbs an average of 48 lbs of CO2 per year.
We give you the chance to participate and be part of this noble project. We plant Trees for you for each product you purchase from SIFSOF.
Let’s re-Green our Earth together? ![]()
![]()
![]()